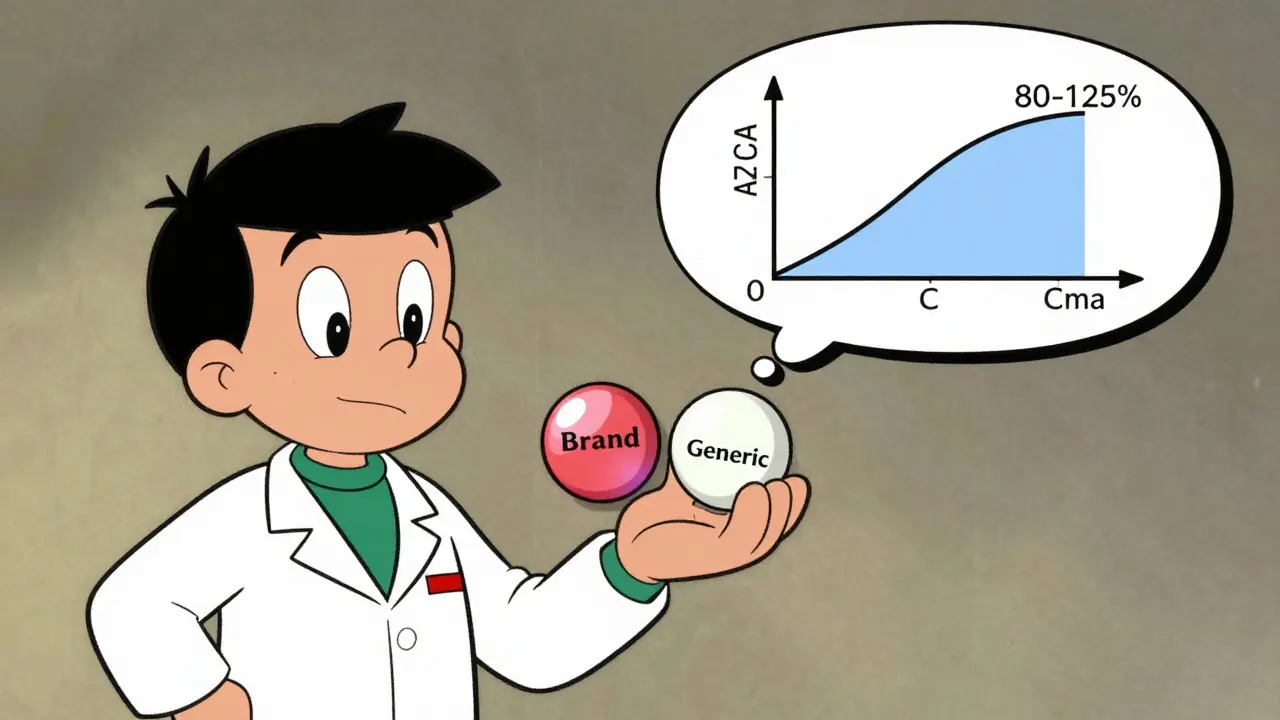
How to Compare Bioavailability and Bioequivalence Concepts in Generic vs Brand Drugs
Bioavailability measures how much of a drug enters your bloodstream, while bioequivalence compares generic and brand drugs to ensure they deliver the same amount at the same rate. Learn how regulators use AUC and Cmax to approve generics safely.