
When a brand-name drug loses its patent, prices usually crash. Patients expect cheaper pills. Competitors rush in to make copies. But what if the brand company itself starts selling the generic version? It sounds strange, but it’s real - and it’s happening more than you think.
What Exactly Is an Authorized Generic?
An authorized generic isn’t a knockoff. It’s the exact same pill, made in the same factory, with the same ingredients, same coating, same everything - just without the brand name on the box. The company that invented the drug - say, Pfizer or Eli Lilly - produces it under a different label and sells it as a generic. The FDA calls these products "identical" to the brand version in every way except packaging and name.Why does this matter? Because when a patent expires, generic manufacturers can legally copy the drug. But they have to prove it works the same way. That takes time, money, and testing. Authorized generics skip all that. The original company already has the data. They’ve already passed every inspection. So they just slap a new label on the same bottles and ship them out.
How Did This Start?
The whole system started with the Hatch-Waxman Act of 1984. Congress wanted to make drugs cheaper without slowing down innovation. So they created a shortcut for generic companies to get approval without repeating expensive clinical trials. But they didn’t stop brand companies from joining the game. In fact, they let them in - quietly.The first big example came in 1997. AstraZeneca was about to lose patent protection for Prilosec, its $6 billion heartburn drug. Instead of watching competitors take over, they launched their own authorized generic under a subsidiary. Within six months, they captured 30% of the market. That’s when other big pharma companies noticed.
How Is It Made?
The process is simple: no new factory, no new formula, no new testing. The same machines that made the brand-name version keep running. The same chemists handle the batches. The same quality checks happen. The only changes are the label, the color of the box, and the name on the bottle.Regulatory approval is faster too. While a regular generic company might wait 17 months for FDA approval, a brand manufacturer can get their authorized generic approved in 6 to 9 months. That’s because they’re using their own existing paperwork - the same data they used to get the original drug approved.
And here’s the kicker: they don’t have to wait for the 180-day exclusivity period that the first generic company gets. That means the brand company can launch on day one - right when the patent expires. In 2019, Teva did exactly that with Copaxone, a multiple sclerosis drug. They launched their own authorized generic on the same day the patent ended and took 22% of the market in just three months.
Why Do Brands Do This?
It’s not about helping patients. It’s about protecting profits.When a brand drug goes generic, prices can drop 80% to 85% within a year. That’s a huge hit. By launching their own authorized generic, companies keep a slice of that market. They don’t lose everything. They might sell the branded version at full price to loyal customers, then offer the authorized generic at a slight discount - say, 10% to 15% cheaper - to price-sensitive buyers.
Data from Drug Patent Watch shows that when a brand company launches an authorized generic, they usually grab 15% to 35% of the generic market in the first year. That means the other generic companies get less. Sometimes, they get almost nothing.
Eli Lilly did this with Cialis. After the patent expired, they introduced their own version. Even though generics flooded the market, Lilly still kept 78% of the total revenue from the drug. That’s not just smart business - it’s a masterclass in market control.
Is This Fair?
Not everyone thinks so.The Federal Trade Commission (FTC) has filed multiple antitrust cases against companies for using authorized generics to block competition. In one case against Actavis over the drug Namenda, the FTC won a $448 million settlement. The argument? That launching your own generic isn’t competition - it’s a way to scare off other companies before they even start.
Dr. Aaron Kesselheim from Harvard Medical School says authorized generics give the illusion of choice but deliver little real savings. His research found that in markets with authorized generics, prices dropped only 32%. In markets without them, prices dropped 68%. That’s a huge difference.
On the flip side, the pharmaceutical industry argues that authorized generics increase access. They say patients get the same drug they’ve always trusted - just cheaper. And the FDA confirms: 99.7% of authorized generics are bioequivalent to the brand version. So, medically, they’re identical.
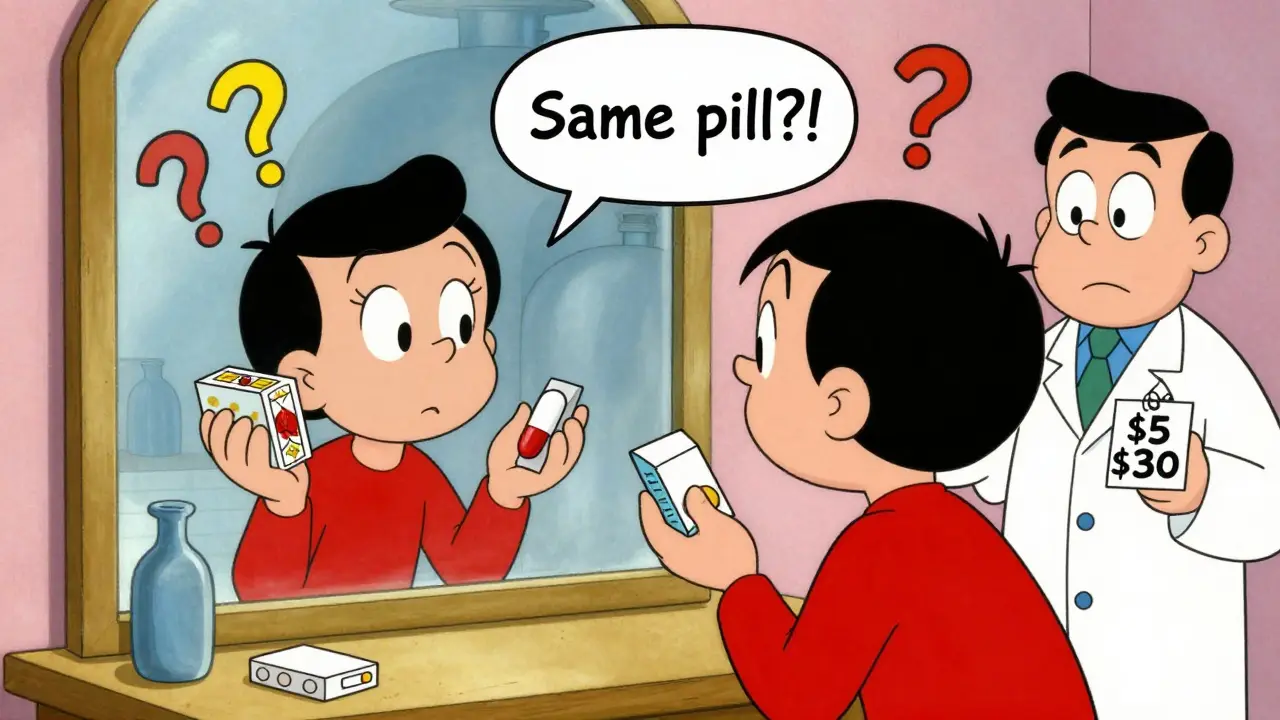
What Do Patients Think?
Patients are confused.On Reddit, users in r/pharmacy posted hundreds of comments wondering: "If it’s the same pill, why is it called generic?" Many noticed that the authorized generic was only $5 cheaper than the brand, while other generics were $30. They felt tricked.
Pharmacists say patients often don’t realize the authorized generic and the brand are made by the same company. A 2023 Kaiser Family Foundation survey found 71% of patients preferred authorized generics - but 64% didn’t know they were made by the brand manufacturer.
Still, reviews on Drugs.com show higher satisfaction for authorized generics than for regular generics. People write things like: "This is the exact same pill I’ve been taking for 10 years. No side effects. No surprises. I trust it." That’s powerful.
The Future: Biologics and Beyond
The next wave is coming - and it’s even more complex.Biologic drugs - like insulin, cancer treatments, and autoimmune therapies - are made from living cells, not chemicals. Copying them is harder. That’s why biosimilars (the generic version of biologics) take longer to develop and cost more.
But now, companies are starting to make authorized biosimilars. In 2023, Amgen launched the first one: their own version of Enbrel, a rheumatoid arthritis drug. It’s the same drug, same factory, same process - just labeled differently.
Analysts predict that by 2025, 40% of small-molecule drugs losing patents will have authorized generics from the original maker. For biologics, that number could be even higher.
What This Means for You
If you’re taking a brand-name drug and it just went generic, check the label. Is the manufacturer name the same as the brand? That’s likely an authorized generic.You might pay less. But you might not pay much less. And you might not realize you’re still buying from the same company.
Ask your pharmacist: "Is this made by the brand company?" If they say yes, you’re getting the same pill - just without the fancy packaging. If you’re cost-conscious, compare it to other generics. Sometimes, the real savings come from the ones made by independent companies.
Either way, you’re getting a safe, FDA-approved product. But knowing who made it - and why - helps you make smarter choices.
Are authorized generics the same as regular generics?
Yes and no. Authorized generics are made by the original brand company using the same formula, factory, and quality controls as the brand-name drug. Regular generics are made by other companies that reverse-engineer the drug. Both are FDA-approved and bioequivalent, but authorized generics are exact copies - down to the color and shape - while regular generics can look different.
Why is an authorized generic sometimes more expensive than a regular generic?
Because the brand company doesn’t want to undercut itself. They price authorized generics just low enough to attract customers who want to save money, but high enough to protect their brand-name sales. Regular generics, made by competing companies, often compete on price and can drop much lower - sometimes 80% cheaper than the brand.
Can I trust an authorized generic as much as the brand name?
Absolutely. The FDA requires authorized generics to be identical in active ingredients, strength, dosage form, and performance. They’re made in the same facility under the same strict rules. Many patients report no difference in how they feel. The only thing that changes is the label.
Do authorized generics delay cheaper generics from entering the market?
Yes, that’s a major criticism. By launching their own generic on day one, brand companies can capture market share before other generic makers even get started. This reduces the financial incentive for competitors to enter, and sometimes delays their launch. The FTC has taken legal action against companies for using this tactic to block competition.
How can I tell if my generic drug is an authorized generic?
Look at the manufacturer name on the bottle. If it’s the same company that makes the brand-name version - like Pfizer, Eli Lilly, or AstraZeneca - it’s an authorized generic. You can also ask your pharmacist directly. Some pharmacies label them as "authorized generic" on the receipt or in their system.




Carolyn Benson
December 20, 2025So the pharma giants just slap a new label on the same pills and call it a "generic"? Brilliant. They get to keep the profits, confuse patients, and still act like they're doing us a favor. This isn't capitalism. It's a rigged game where the house always wins - and we're the suckers paying for the chips.
They don't care if you save money. They care if you keep buying from them. Even when you think you're avoiding the brand, you're still handing them cash. It's psychological manipulation wrapped in FDA approval.
I used to trust generics. Now I check the manufacturer. If it's Pfizer, Eli Lilly, or any of those names - I walk away. I'd rather pay the brand price than fund their monopoly games.
And don't get me started on the "bioequivalent" lie. Same ingredients doesn't mean same experience. Fillers change. Coatings change. Your body notices. You just don't know why you feel off.
They've turned healthcare into a shell game. And the FTC? They're still playing catch-up while patients get fleeced every month.
It's not innovation. It's exploitation dressed in white coats.
Erica Vest
December 20, 2025Authorized generics are legally identical to brand-name drugs under FDA regulations - same active ingredients, same manufacturing process, same quality controls. The only difference is the label and packaging.
They're not a loophole. They're a regulatory option explicitly allowed under Hatch-Waxman. The FDA requires bioequivalence testing for all generics, but authorized generics skip that because they're the original product.
For patients, this means zero risk. No variability. No guesswork. Just the same pill you've been taking, at a slightly lower price.
It's not deception. It's efficiency. Why force a new company to replicate what already exists when the original maker can do it faster, safer, and cheaper?
Yes, it reduces competition - but it also increases access. More people get the drug they need. That’s not evil. That’s logistics.
Isabel Rábago
December 20, 2025People act like this is some new scam, but it’s been going on since the 90s. You want to be mad? Be mad at Congress for letting this loophole exist. Be mad at the FDA for greenlighting it. Be mad at your doctor for never telling you.
But don’t act surprised. Pharma doesn’t give a damn about you. They care about profit margins. If they can keep 30% of the market by selling the exact same pill under a different name, they will. Every time.
And you? You’re just another number on a balance sheet. You think you’re saving money? You’re just paying less to the same thief.
It’s not a market. It’s a prison with a discount coupon.
Mike Rengifo
December 22, 2025My grandma takes Lipitor. She switched to the generic and saved $40 a month. Last month she noticed the manufacturer was Pfizer. She called me freaking out. "Are they lying to me?"
I told her it’s the same pill. She still didn’t trust it. Said she’d rather pay more for the blue pill with the big logo.
Turns out she wasn’t dumb. She was wise. She knew who she was dealing with.
People don’t hate generics. They hate being played.
holly Sinclair
December 23, 2025What’s fascinating here isn’t just the corporate maneuvering - it’s the epistemological collapse of trust in medicine.
We’ve been taught that generics are cheaper, inferior, risky alternatives. But authorized generics shatter that narrative. They’re not inferior. They’re identical. So why do we still think of them as "second-class"? Because branding isn’t about the pill - it’s about the story.
The brand tells us: "This is safe. This is reliable. This is worth paying for." The generic says: "This is cheaper." But when the same company makes both, the story fractures.
Now we’re left wondering: Is the drug the product? Or is the brand the product? And if the brand is the product, then what are we really buying when we buy a pill?
And if the pill is identical, why does the label matter so much? Why do we trust the name more than the chemistry?
This isn’t just about pharmaceuticals. It’s about how capitalism turns trust into currency - and how we’ve all learned to pay for labels instead of substances.
Maybe the real scandal isn’t that they do this - it’s that we still let them get away with it.
Emily P
December 23, 2025My pharmacist told me my generic was an authorized one. I didn’t even know that was a thing. I asked why it cost $15 instead of $5 like the other one. She said, "Because it’s the same pill. The company just put a different box on it."
I felt weird. Like I got scammed even though I didn’t.
Now I always ask. And honestly? I’d rather pay the $5 generic from some unknown company than give money to the same corporation that charged me $400 for the brand.
It’s not about the pill. It’s about who made it.
Chris Davidson
December 25, 2025Stop acting like this is a conspiracy. The law allows it. The FDA approves it. The market responds to it. If you don’t like it then buy the brand or go without. Simple.
People want cheap drugs but also want to be coddled. They want the same pill but complain when it’s made by the same company. That’s not logic. That’s emotional whining.
Authorized generics are a win for patients who want consistency. The rest of you are just mad because you can’t blame someone else for your own lack of research.
Matt Davies
December 26, 2025Imagine if your favorite coffee shop started selling the exact same beans in plain brown bags for half the price. You’d think, "Oh cool, savings!" But then you realize - it’s still the same owner. Same roaster. Same beans. Just no logo.
That’s this. And honestly? I’m okay with it. I’d rather have the same quality I know, even if the company is still profiting.
It’s not perfect. But it’s better than waiting 18 months for some startup to reverse-engineer a pill and then getting inconsistent batches.
Let’s not pretend we’re saving the world by choosing a $3 generic. Sometimes, the "real" generic is the one with the brand’s name on the back of the bottle.
benchidelle rivera
December 27, 2025Let me be clear: this isn’t about choice. It’s about control. The brand companies aren’t competing - they’re co-opting. They’re using their own patents and regulatory access to strangle competition before it even breathes.
They don’t care if you save money. They care if you don’t have options.
And they’re not alone. This is how every industry works now - Amazon with private labels, Apple with refurbished models, Netflix with their own versions of shows studios made.
They don’t just enter the market. They own the market. And we’re just supposed to be grateful they let us buy from them.
It’s not capitalism. It’s feudalism with a pharmacy counter.
bhushan telavane
December 29, 2025In India we call this "copycat brand" - big pharma makes the original, then sells the exact same thing under a local name. We know it’s the same. But we still buy the brand because it feels safer.
Same here. Americans don’t trust generics because they don’t know who made them. But if it says Pfizer? They’ll buy it even if it’s $10 more.
It’s not about science. It’s about branding. And that’s the real drug.
Alex Curran
December 31, 2025My endocrinologist told me to switch from brand insulin to the authorized generic. I was nervous. But after 6 months? Zero side effects. Same dose. Same results.
It saved me $120/month. I’m not mad. I’m grateful.
Yes, the company still made money. But I got the same life-saving drug at a price I can afford.
Stop acting like every corporate move is evil. Sometimes the system works - even if it’s messy.
Focus on access. Not blame.
Mahammad Muradov
January 1, 2026Anyone who thinks this is fair hasn’t studied antitrust law. This is textbook predatory pricing disguised as consumer benefit. The FTC has documented this pattern for 20 years. Companies use authorized generics to kill competitors before they even launch.
It’s not innovation. It’s extortion.
The FDA doesn’t care about market dynamics. They only care if the pill works. But that’s not the issue. The issue is who gets to make it and who gets to survive.
This is how monopolies are maintained. And you’re helping them by buying it.
Chris porto
January 1, 2026It’s weird how we get mad at corporations for doing exactly what we tell them to do.
We want cheaper drugs. So they make a cheaper version.
We want the same quality. So they use the same factory.
We want it fast. So they skip the redundant testing.
Then we act surprised when they profit from it.
It’s not a trap. It’s a system. And we’re all part of it.
Maybe the real question isn’t why they do it - but why we keep letting them.
Jedidiah Massey
January 2, 2026Authorized generics are the pinnacle of neoliberal pharmaceutical strategy: extractive efficiency wrapped in regulatory compliance. The firm leverages its IP infrastructure to preempt market entry by third-party generics, thereby internalizing the externalized cost of R&D while maintaining price elasticity through tiered pricing architectures.
Essentially, they’re deploying a dual-market penetration strategy - premium branding for loyalists, authorized generics for elasticity-sensitive segments - optimizing for shareholder value while maintaining the illusion of competition.
It’s not unethical. It’s optimal.
Also, if you’re paying more than $5 for a generic, you’re being played. 😎
Kinnaird Lynsey
January 3, 2026Look. I get why people are upset. But I also get why I buy the authorized generic.
I’ve had bad reactions to other generics. Not because they’re unsafe - but because the fillers or coatings are different. My body remembers the brand. It’s not irrational. It’s biological.
So I pay $10 extra for the one that’s literally the same pill.
Am I being manipulated? Maybe.
But I’d rather be manipulated by the company I’ve trusted for 15 years than gamble on a $3 pill from a factory I’ve never heard of.
It’s not about justice. It’s about peace of mind.
And sometimes… that’s worth a little more.