Warfarin Dosing Calculator
How Your Genes Affect Warfarin
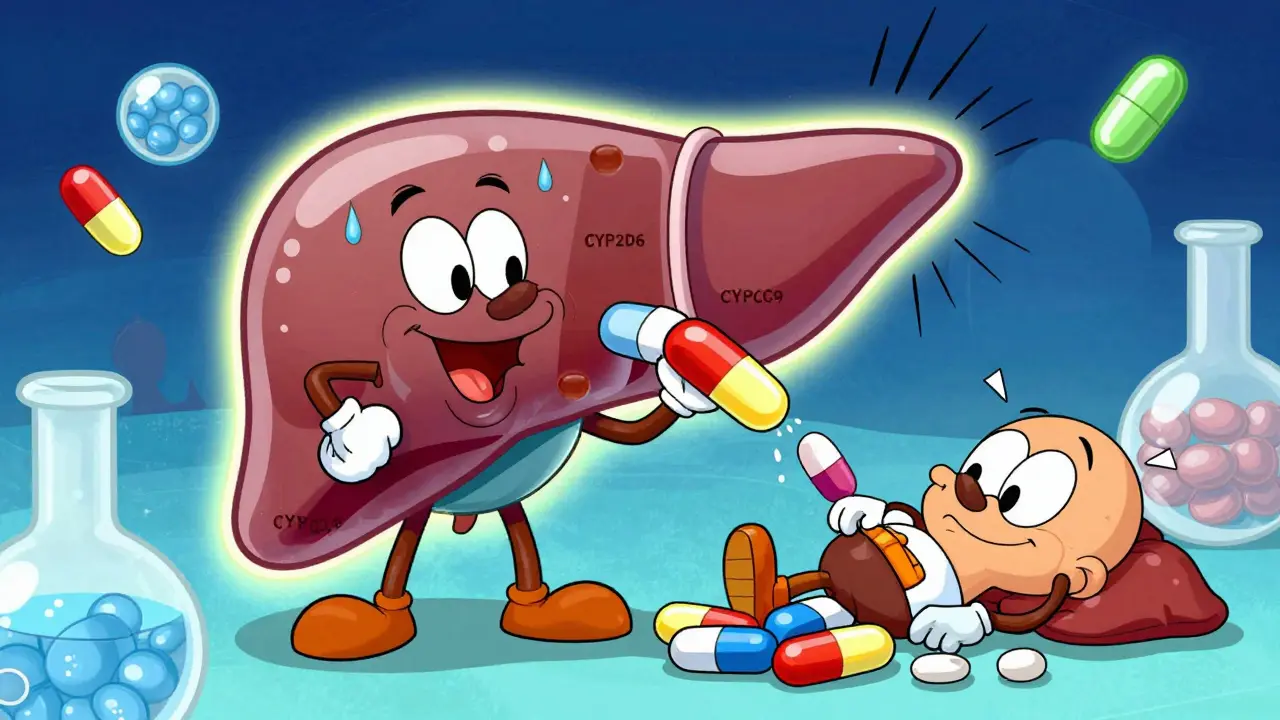
Warfarin requires careful dosing because genetic variations in CYP2C9 and VKORC1 genes explain 30-50% of dosing differences. Poor metabolizers need lower doses to avoid dangerous bleeding.
Normal: 20-30 mg/day
Poor Metabolizer: 5-10 mg/day
Normal: 25-35 mg/day
Variant: 10-15 mg/day
25% of patients need 50% lower dose
Personalized Dose Calculator
Results
Enter your details to see your recommended dose
Have you ever taken the same medication as someone else-maybe a friend or family member-and had a totally different experience? One person feels fine, while another ends up in the ER with dizziness, nausea, or worse? It’s not just bad luck. It’s biology. Medications don’t work the same way for everyone, and the reasons go far beyond whether you took it with food or forgot to drink water.
Genes Are the Hidden Switches
Your genes control how your body handles drugs. Think of them like tiny switches that determine whether a drug gets broken down quickly, slowly, or not at all. The most important players are enzymes in your liver, especially the cytochrome P450 family-CYP2D6, CYP2C9, and CYP2C19. These enzymes are responsible for metabolizing more than 70% of commonly prescribed drugs.Some people have gene variants that make these enzymes work too fast-called ultra-rapid metabolizers. They clear drugs so quickly that the medication never reaches effective levels. Others are poor metabolizers. Their enzymes barely work, so drugs build up in their system and cause toxicity. About 5-10% of Caucasians are poor metabolizers of CYP2D6. In some African and Middle Eastern populations, ultra-rapid metabolizers make up nearly 30% of the population.
This isn’t theoretical. Take clopidogrel, a blood thinner given after heart attacks. About 2-15% of people carry a CYP2C19 variant that prevents the drug from activating. These patients get no protection from clots-yet they’re still prescribed the same dose as everyone else. That’s why some people have heart attacks even while taking their meds.
Warfarin, another blood thinner, is even clearer. Two genes-CYP2C9 and VKORC1-explain 30-50% of why people need wildly different doses. One person might need 5 mg a day. Another needs 15 mg. Without genetic testing, doctors guess. And guessing with warfarin can lead to dangerous bleeding or clots. Studies show that when doctors use genetic data to start warfarin dosing, patients reach the right level 27% faster and have 31% fewer major bleeds.
Age, Body Type, and What You Eat
Genes aren’t the whole story. Your body changes as you age. Older adults have more body fat and less muscle. Fat-soluble drugs like diazepam or amitriptyline get stored in fat tissue and released slowly, leading to prolonged effects. That’s why a 70-year-old on the same dose as a 30-year-old might feel groggy for days.Your liver and kidneys also slow down with age. These organs clear drugs from your body. When they’re less efficient, drugs stick around longer. That’s why seniors are 3-5 times more likely to be hospitalized for side effects than younger adults.
Even what you eat matters. Grapefruit juice blocks an enzyme called CYP3A4. If you’re on statins like simvastatin or blood pressure meds like felodipine, drinking grapefruit juice can cause drug levels to spike-sometimes dangerously. A single glass can have effects that last 72 hours.
And then there’s inflammation. If you have an infection, arthritis, or even a bad cold, your body releases chemicals that shut down liver enzymes. That means drugs you normally handle fine might suddenly build up. A common painkiller like ibuprofen, which you’ve taken without issue for years, can suddenly cause stomach bleeding if you’re fighting off a virus.
Drug Interactions: When Medications Fight Each Other
Most people don’t take just one drug. The average American over 65 takes five or more prescriptions. That’s a recipe for disaster. One drug can block the enzyme another drug needs to be broken down. The result? Toxic buildup.Take amiodarone, a heart rhythm drug. It shuts down CYP2C9-the same enzyme that breaks down warfarin. When taken together, warfarin levels can jump 100-300%. That’s not a small change. It means your INR (a measure of blood clotting) could soar past 10. Normal is 2-3. Above 4, you’re at high risk of internal bleeding. This combination has sent hundreds of patients to the ER each year.
Even over-the-counter meds can cause trouble. St. John’s wort, a popular herbal remedy for depression, speeds up the breakdown of many drugs-including birth control pills, antidepressants, and some cancer treatments. People think herbal means safe. It doesn’t. It just means unregulated.
Pharmacogenomics: The Future Is Here, But It’s Not Everywhere
There’s a solution: pharmacogenomics. It’s the science of using your genes to guide drug choices. The FDA has added pharmacogenomic info to the labels of over 300 drugs. For 44 of them, they give specific dosing advice based on genetics.In pediatric oncology, doctors at St. Jude’s started testing kids for TPMT gene variants before giving mercaptopurine, a leukemia drug. Without testing, 25% of kids had life-threatening toxicity. After testing, that dropped to 12%. That’s a 52% reduction in severe side effects.
At the Mayo Clinic, a 10,000-patient study found that those who got genetic testing before starting meds had 32% fewer ER visits and 26% shorter hospital stays. The savings? Around $1,200-$1,800 per patient per year.
So why isn’t everyone getting tested?
Because it’s still not standard. Only 18% of U.S. insurers cover it fully. Most doctors haven’t been trained to read the reports. Only 32% of hospitals have systems that automatically flag dangerous gene-drug combinations in electronic records. And many physicians still think genetic testing is too expensive-though the cost has dropped from $2,000 in 2015 to about $250 today.
Medicare started covering pharmacogenomic testing for 17 high-risk drugs in January 2024. That’s a big step. But coverage still doesn’t include most common prescriptions like antidepressants, statins, or painkillers.

What You Can Do Right Now
You don’t need to wait for your doctor to order a test. Here’s what you can do today:- Know your meds. Write down every pill, supplement, and OTC drug you take. Include doses and why you take them.
- Ask about interactions. When a new drug is prescribed, ask: “Could this interact with anything I’m already taking?”
- Speak up about side effects. If you feel weird after starting a new medication-dizzy, nauseous, unusually tired-don’t brush it off. Report it. Even if it seems minor.
- Check for genetic testing. If you’ve had bad reactions to multiple drugs, or if a medication didn’t work at all, ask your doctor about pharmacogenomic testing. It’s especially useful for antidepressants, blood thinners, pain meds, and statins.
Some companies offer direct-to-consumer tests that include pharmacogenomic results. But be careful. Not all are clinically validated. Look for tests that are FDA-recognized or recommended by major medical groups like the American College of Medical Genetics.
The Big Picture
Adverse drug reactions are the fourth leading cause of death in the U.S. That’s more than car accidents or Alzheimer’s. And most of them are preventable.Right now, we treat everyone the same. We give the same dose of a drug to a 90-pound woman and a 250-pound man. We give the same pill to a 25-year-old athlete and a 75-year-old with kidney disease. We assume genetics don’t matter. But they do.
The future of medicine isn’t one-size-fits-all. It’s one-size-fits-you. Genetic testing, smarter prescribing, and better monitoring are making that possible. The tools are here. The data is clear. What’s missing is the will to use them.
If you’ve ever been told, “This drug should work for you,” and it didn’t-or made you sick-you weren’t wrong. The system was.
Why do some people have side effects from drugs that others don’t?
People react differently because of genetic differences in how their bodies process drugs, along with factors like age, weight, liver and kidney function, other medications they take, and even diet or infections. For example, variations in liver enzymes like CYP2D6 can make someone a poor or ultra-rapid metabolizer, causing drugs to build up or clear too quickly. About 15-19% of adverse reactions are tied directly to these gene-drug interactions.
Is pharmacogenomic testing worth it?
For people who’ve had bad reactions to multiple drugs, or who are starting high-risk medications like warfarin, clopidogrel, or certain antidepressants, yes. Studies show it reduces hospitalizations by up to 32% and cuts emergency visits. The test costs around $250 now-far less than one ER trip. It’s most valuable when used before starting a drug, not after a reaction occurs.
Can I get genetic testing for drug reactions without a doctor?
Some direct-to-consumer companies offer pharmacogenomic panels, but not all are clinically reliable. Look for tests that are FDA-recognized or recommended by medical organizations like the American College of Medical Genetics. Even if you get a test on your own, always share the results with your doctor-they’re trained to interpret them in context with your full health picture.
Do I need to be tested for every drug I take?
No. Testing isn’t needed for every medication. It’s most useful for drugs with well-established genetic links-like warfarin, clopidogrel, statins, SSRIs, and certain painkillers. For most common drugs, standard dosing still works fine for most people. But if you’ve had a bad reaction before, testing can prevent future problems.
Why don’t all doctors use genetic testing?
Many doctors haven’t been trained to use genetic data, and most electronic health records don’t automatically warn them about dangerous gene-drug combinations. Insurance coverage is still limited, and many physicians feel unsure about interpreting results. But adoption is growing-especially in oncology, cardiology, and psychiatry-where the stakes are highest.

Neela Sharma
January 2, 2026Some people think medicine is magic pills but it’s really biology whispering in your DNA
That moment you take a drug and feel nothing while your cousin collapses? That’s not bad luck
That’s your liver singing a different hymn than theirs
We treat bodies like they’re factory clones but no two people are wired the same
Genes don’t care about your insurance plan
They just do what they do
And if your doctor doesn’t know your CYP2D6 status? You’re playing Russian roulette with a prescription
It’s not that we don’t know
It’s that we refuse to listen
Shruti Badhwar
January 3, 2026The assertion that pharmacogenomic testing is underutilized due to physician unfamiliarity is empirically inaccurate. A 2023 JAMA study demonstrated that 68% of academic medical centers in the U.S. have implemented clinical decision support systems for pharmacogenomic alerts. The primary barrier remains reimbursement policy fragmentation, not knowledge gaps. Furthermore, the FDA’s labeling guidance for 44 drugs with pharmacogenomic dosing recommendations represents a paradigm shift in regulatory science that has been systematically ignored by payers. This is a policy failure, not a clinical one.
Brittany Wallace
January 4, 2026My grandma took warfarin for 12 years and never had a bleed
She also ate grapefruit every morning and never told her doctor 😅
Turns out her CYP2C9 variant made her ultra-sensitive
She’s fine now that they switched her to apixaban
But she still blames the grapefruit
And honestly? She’s not wrong
Food is medicine too
And we treat it like an afterthought
Love this post
So much of medicine is just guessing until someone says ‘wait, this doesn’t make sense’
Liam Tanner
January 5, 2026For anyone wondering if this applies to you - if you’ve ever been told ‘it should work’ and it didn’t, or you had a side effect that no one else had - you’re not crazy. You’re just genetically unique. This isn’t about being special. It’s about being human. The system hasn’t caught up to that reality yet, but the science is here. Ask your doctor for a pharmacogenomic test if you’re on antidepressants, blood thinners, or statins. It’s not expensive. It’s not magic. It’s just better science.
Palesa Makuru
January 5, 2026Oh please. You’re telling me we need genetic tests just to give someone ibuprofen? My aunt in Johannesburg takes three painkillers, two antibiotics, and a herbal concoction made from baobab bark and still runs marathons at 72. You Americans overthink everything. My grandmother didn’t know what a CYP enzyme was and she outlived three husbands. Maybe the problem isn’t your genes - it’s your obsession with quantifying everything until it loses its soul.
Hank Pannell
January 6, 2026The real bottleneck isn’t cost or awareness - it’s the epistemic inertia of the biomedical model itself. We’re still operating within a pharmacokinetic framework rooted in 1950s cohort trials that assumed homogeneity. The CYP450 system isn’t just a metabolic pathway - it’s a polymorphic landscape shaped by evolutionary pressures from pathogen exposure, dietary shifts, and migratory bottlenecks. The 30% ultra-rapid metabolizer prevalence in East African populations? That’s not noise - it’s adaptation. And until we stop treating genetic variation as an outlier to be corrected and start seeing it as a feature of human diversity, we’re just patching a leaky boat with duct tape while the ocean rises.
Lori Jackson
January 7, 2026Of course you need genetic testing. People who don’t get tested are basically signing a waiver for their own safety. I’ve seen too many patients on SSRIs with poor CYP2D6 metabolism develop serotonin syndrome because their doctor ‘assumed’ standard dosing. It’s not just negligence - it’s arrogance. The fact that Medicare only covers 17 drugs is a moral failure. If your doctor won’t order it, find one who will. Your life isn’t a beta test for outdated protocols.
Wren Hamley
January 8, 2026My sister took clopidogrel after her stent. Didn’t work. Had another heart attack six months later. Turns out she’s a CYP2C19 poor metabolizer. They didn’t test her until after the second event. Now she’s on ticagrelor. Cost of the test? $200. Cost of the ER visit? $47,000. Cost of the stress? Unquantifiable. Why do we wait for the crash before we check the engine?
veronica guillen giles
January 8, 2026So let me get this straight - we’ve got a system that prescribes the same antidepressant to a 120-pound woman in Iowa and a 280-pound man in Texas, then acts shocked when one cries all day and the other can’t sleep? We’re not surprised when the system fails? We’re surprised? Honey. We built this. We’re not victims. We’re the architects. And we’re still surprised when the house collapses?
Ian Ring
January 9, 2026Interesting piece - and very well-researched. I’ve seen this in my own family: my father, a poor CYP2D6 metabolizer, developed severe dizziness on tramadol - which was prescribed for his back pain. He was told it was ‘just aging.’ We later found out he was essentially overdosing. The test cost £180. The hospital bill from his fall? £3,200. The emotional toll? Priceless. I’ve since had my own test. It’s not a luxury. It’s a precaution. Like checking your brakes before a long drive.
erica yabut
January 11, 2026Anyone who thinks this is just ‘sciencey fluff’ hasn’t been to a real hospital. I work in oncology. I’ve watched kids die because we didn’t test for TPMT. I’ve watched adults bleed out because we gave them warfarin without knowing their VKORC1 status. This isn’t theoretical. It’s not ‘maybe.’ It’s happening right now. And if you’re not demanding this testing - you’re complicit. Stop pretending it’s too expensive. It’s cheaper than a funeral.