Manufacturing Quality Issues in Generic Drugs: What You Need to Know
When you pick up a generic pill, you expect it to work just like the brand-name version. But manufacturing quality issues, flaws in how drugs are produced that can alter their safety or effectiveness mean that’s not always true. These aren’t rare mistakes—they happen in factories that supply millions of pills, and they can change how your body absorbs the medicine. Even small differences in how a drug is pressed, coated, or blended can make it fail to work—or worse, cause harm.
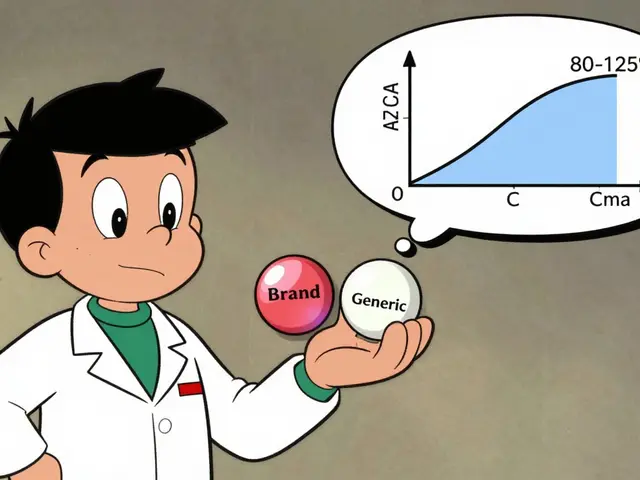
generic drugs, lower-cost copies of brand-name medications approved by the FDA are supposed to match the original in strength, purity, and performance. But when bioequivalence, the measure of whether a generic drug delivers the same amount of active ingredient at the same rate as the brand isn’t properly tested or enforced, the results can be dangerous. For drugs with a narrow therapeutic index, medications where the difference between a safe dose and a toxic one is very small—like lithium, warfarin, or thyroid meds—even a 5% variation in absorption can trigger seizures, blood clots, or thyroid crashes. And it’s not just about the active ingredient. Fillers, binders, and coatings matter too. A change in one of those can make a pill dissolve too fast, too slow, or not at all.
Some of the worst cases have come from overseas factories cutting corners—skipping cleanroom protocols, using substandard ingredients, or falsifying test results. The FDA inspects foreign plants less often than U.S. ones, and when problems are found, recalls are often delayed. You might not know your generic was made in a facility with a history of violations. That’s why people on critical meds track their pharmacy’s source, ask about batch numbers, and watch for sudden side effects after a refill.
This collection of articles dives into real cases where manufacturing quality issues led to treatment failure, hospital visits, or even deaths. You’ll find stories about lithium generics that sent patients into seizures, statins that didn’t lower cholesterol, and antibiotics that failed to treat infections—all because the pills didn’t perform as they should. We’ll show you how to spot red flags, what questions to ask your pharmacist, and how to use the FDA’s public databases to check a drug’s history. You don’t have to guess whether your medicine is safe. There are tools, tricks, and truths you can use right now to protect yourself.