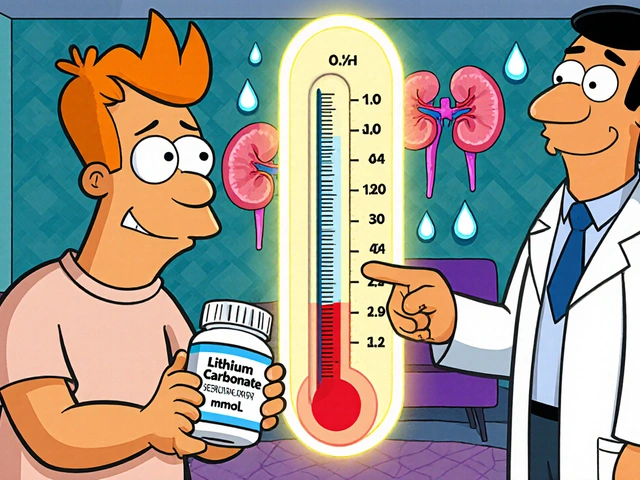
Generic Drug Defects: When Cheap Medicines Fail You
When you pick up a generic drug, a lower-cost version of a brand-name medication that must meet FDA standards for safety and effectiveness. Also known as generic medication, it's supposed to be just as good as the original—but sometimes, it isn't. That’s not a rumor. It’s a documented problem, especially with drugs that have a narrow therapeutic index, a range so small between the effective dose and the toxic dose that tiny changes in absorption can cause serious harm. Think lithium for bipolar disorder, warfarin for blood thinning, or levothyroxine for thyroid function. A slight shift in how your body absorbs the drug—because of a different filler, coating, or manufacturing process—can throw your whole treatment off track.
These aren’t rare edge cases. In 2021, the FDA confirmed cases where generic versions of levothyroxine caused patients to go from stable to hyperthyroid after a switch. The same happened with generic versions of seizure meds and antidepressants. Why? Because bioequivalence, the legal standard that says a generic must absorb into the bloodstream within 80–125% of the brand-name version doesn’t guarantee identical results in real life. That 45% range? It’s wide enough for side effects to spike or疗效 to drop. And when you’re on a daily med for a chronic condition, even a 5% difference adds up over weeks and months.
It’s not just about chemistry. drug quality, how consistently a product is made across batches and over time varies between manufacturers. Some factories overseas cut corners—using inferior binders, skipping stability tests, or failing to control humidity during production. These aren’t illegal drugs. They’re approved by the FDA. But approval doesn’t mean perfection. And when your blood levels start to drift, your doctor might blame you for noncompliance, when the real issue is the pill in your hand.
So what can you do? Track your symptoms. If you switch generics and suddenly feel worse, dizzy, anxious, or your condition worsens, speak up. Ask for the brand name or a specific generic manufacturer. Keep a log of when you switch pills and what happens. Use the FDALabel database, the official source for full drug labeling, including warnings about generic variations to check if your drug has a history of complaints. And if your insurance forces a switch, know your rights—you can appeal.
This collection of articles dives deep into the real-world fallout of these issues. You’ll find stories of people whose lithium levels crashed after a generic switch, how certain diabetes drugs trigger deadly infections, why some antidepressants cause withdrawal even when you taper slowly, and how to spot a faulty prescription label before it harms you. These aren’t hypotheticals. They’re lived experiences. And if you’re taking a generic drug—especially one with a narrow therapeutic window—you need to know what to watch for. The next pill you take might be safe. But it might not be. And you deserve to know why.